Training
CITI Program
Most necessary trainings can be taken through CITI Program at about.citiprogram.org
- For new users, please select the "Register" button at the top right. CITI has a guide and video to help you get started.
- In the first question, please affiliate with "Missouri State University".
- In the "Primary Email" use your abc123s@missouristate.edu email address (do not use login).
- Continue to fill in your information accordingly.
Already have a CITI account? You can add new courses using the "Add a Course" option at the bottom of the "My Courses" tab.
Explanation of classes can be found in the drop-down menu below.
-
As MSU faculty, students, or staff members involved in the conduct of research applying for federal funding, you will be required to take compliance training on a variety of topics. Once the grant is awarded, yearly training may be mandated by the agency for the life of the grant. See the below descriptions.
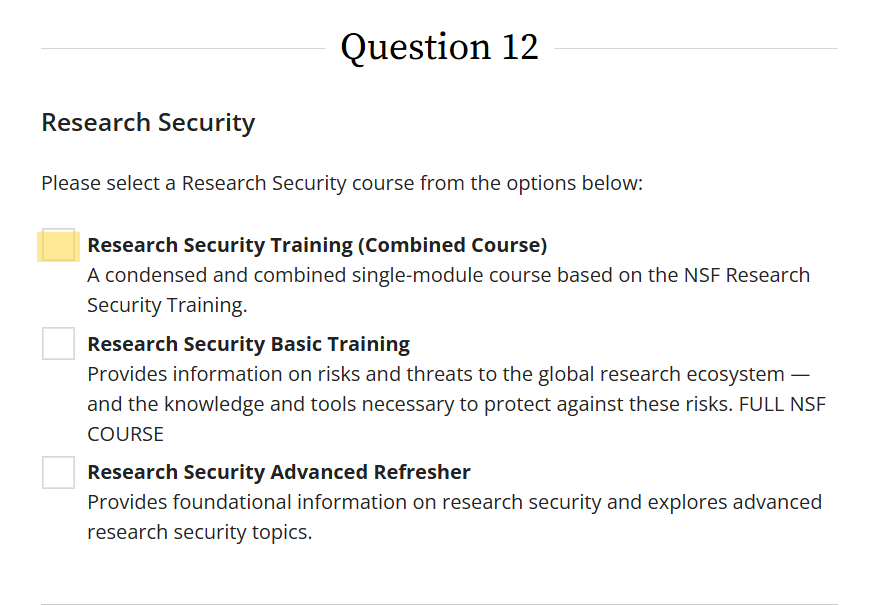
1. Research Security training - found in question 12 in CITI. Please select the first option "Research Security Training (Combined Course)".
Who- All Principal Investigators and key personnel need to complete this training within 12 months prior to any Federal research grant application deadline (NSF, USDA, FDA, DOE*, DOD, NIH, NASA). Any key or senior personnel added after the award will need to complete the training within 12 months of joining.
*US Department of Energy
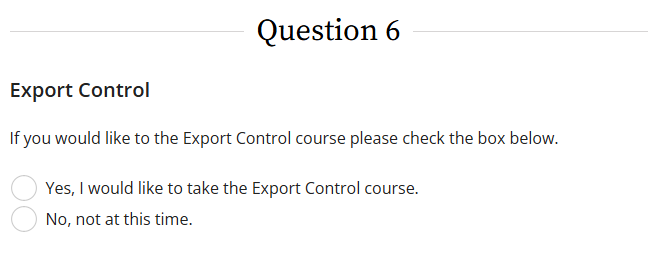
2. Export Control training - found in question 6 in CITI.
Who- All Principal Investigators and key personnel need to complete this training within 12 months prior to any Federal research grant application deadline (NSF, USDA, FDA, DOE, DOD, NIH). All other research personnel, post docs, graduate students, and undergraduate student researchers need to complete the training before they can begin working on the grant.
3. Responsible Conduct of Research (RCR) training - found in question 3 in CITI
Who- All Principal Investigators, key personnel, research personnel, post docs, graduate students, and undergraduate student researchers need to complete RCR training before they can begin working on the grant.

4. Conflict of Interest training (PHS Requirement) - found in question 4 in CITI
Who- All PHS funded research grants need to complete the training. NIH, FDA, CDC, ACF, AoA, CMS, AHRQ, ATSDR, FOH, HRSA, HIS, SAMHSA, and Public Health Service Commissioned Corps are PHS agencies. Those receiving funding from Missouri Department of Health and Senior Services and other state health agencies that include federal funding, will also need to complete training.
Who- PI and key personnel will need to take the training prior to award.
Renewal requirement is every 4 years.

5. Essentials of Grant Proposal Development (EGPD)- found in question 9 is not a required course but is available to anyone interested in writing a grant proposal and is recommended by ORA.
-
Human Subjects Training (IRB)
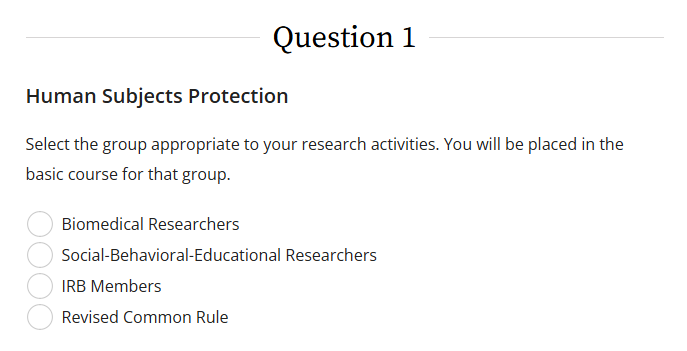
All faculty, staff, and students involved in the design, collection, and/or analysis of data in projects involving human participants must complete the BASIC course of either "Social-Behavioral-Educational Researchers" or "Biomedical Researchers" in question 1 in CITI.
Physical Therapy (PT) students must take Biomedical Research (question 1) per the school's requirement. Anyone in MCHHS who is physically manipulating research participants (taking vitals, bodily fluids, range of motion, etc.) should also complete the Biomedical Researchers course.
Everyone outside MCHHS should take the Social-Behavioral-Educational Researchers training.
Renewal requirement is every 3 years.
Health Information Privacy and Security (HIPS) - found in question 7 in CITI is required for anyone working with Protected Health Information (PHI) from an official health record provided by a covered entity or business associate (a hospital or official health institution).

Researchers working with student data may also be required to complete the Family Educational Rights and Privacy Act (FERPA) training found in question 10. This will be determined on a case-by-case basis depending on the details of your proposed study or if required by federal grant.

Research and Scholarly Activities at Missouri State Universi
-
Animal Research Training (IACUC)
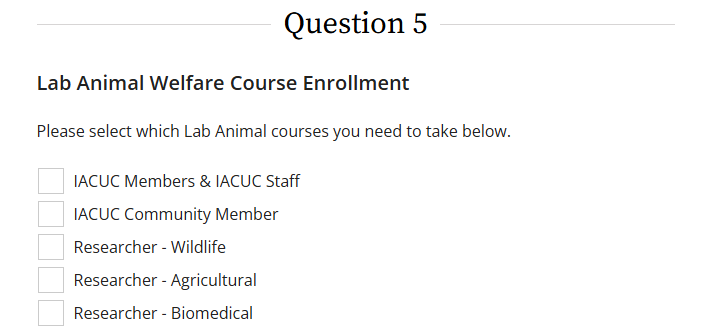
All faculty, staff, and students involved in the design, collection, and/or analysis of data in projects involving animal subjects must complete one of the following trainings found in question 5 dependent on the nature of the proposed study:
- "Researcher - Wildlife" should be taken by anyone studying wild animals, usually Biology students.
- "Researcher - Agricultural" should be taken by anyone studying livestock, usually Agriculture students.
- "Researcher - Biomedical" should be taken by anyone studying laboratory rodents or other laboratory animals, usually Biology or Biomedical students.
Renewal requirement is every 3 years for the researcher courses.
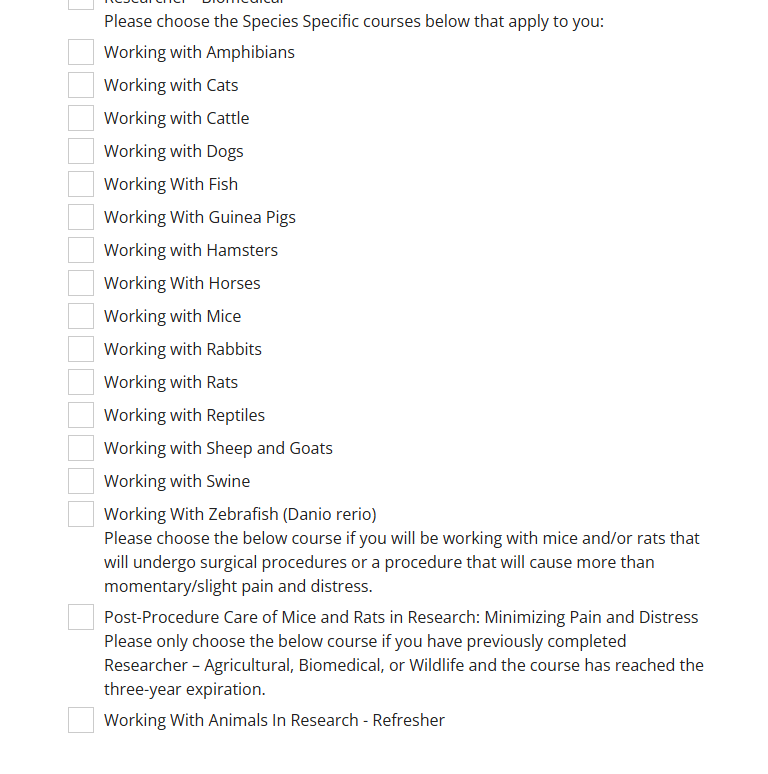
Individual species or groups will also need to be selected. For example if you are working with frogs, you will also need to take the course "Working with Amphibians".
If you are working with horses and cattle, both " Working with __" courses will need to be taken.
If you are working with animals purely in a husbandry capacity, a "Researcher" course will not need to be completed. You will still need to complete the "Working with _____" course(s).
If you are doing surgical procedures on rodents or providing post-op care, "Post-Procedure Care of Mice and Rats in Research: Minimizing Pain and Distress" will be required.
-
Biological Research Training (IBC)
All faculty, staff, and students involved in the design, collection, and/or analysis of data in projects involving biological research (BSL2 Organisms, Synthetic or Recombinant DNA, Bloodborne Pathogens, etc.) must complete the Initial Biosafety Training found in question 8 in CITI.
Renewal requirement is every 2 years.
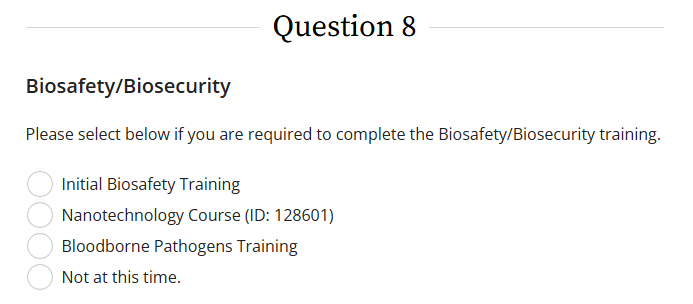
Additionally, researchers may be asked to complete the Nanotechnology Course or Bloodborne Pathogens training also found in question 8 in CITI on a case-by-case basis dependent on the nature of the proposed study.
